![PDF]❤DOWNLOAD⚡ Clinical Trials: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines - Flip eBook Pages 1-1 | AnyFlip PDF]❤DOWNLOAD⚡ Clinical Trials: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines - Flip eBook Pages 1-1 | AnyFlip](https://online.anyflip.com/hxgrd/dcmn/files/mobile/1.jpg?1624012975)
PDF]❤DOWNLOAD⚡ Clinical Trials: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines - Flip eBook Pages 1-1 | AnyFlip
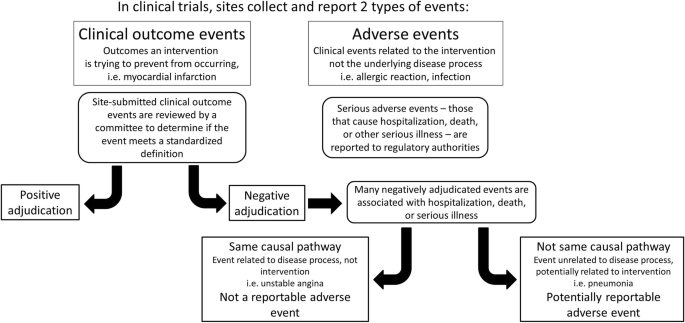
Methods for safety and endpoint ascertainment: identification of adverse events through scrutiny of negatively adjudicated events | Trials | Full Text
On Biostatistics and Clinical Trials: Control for Type I Error (or Adjustment for Multiplicity) for Secondary Endpoints

Clinical Trials: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines - Kindle edition by Brody, Tom. Professional & Technical Kindle eBooks @ Amazon.com.

Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: A randomized clinical trial - Journal of Allergy and Clinical Immunology

Clinical Trials: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines by Tom Brody PhD | NOOK Book (eBook) | Barnes & Noble®
The use of validated and nonvalidated surrogate endpoints in two European Medicines Agency expedited approval pathways: A cross-sectional study of products authorised 2011–2018 | PLOS Medicine
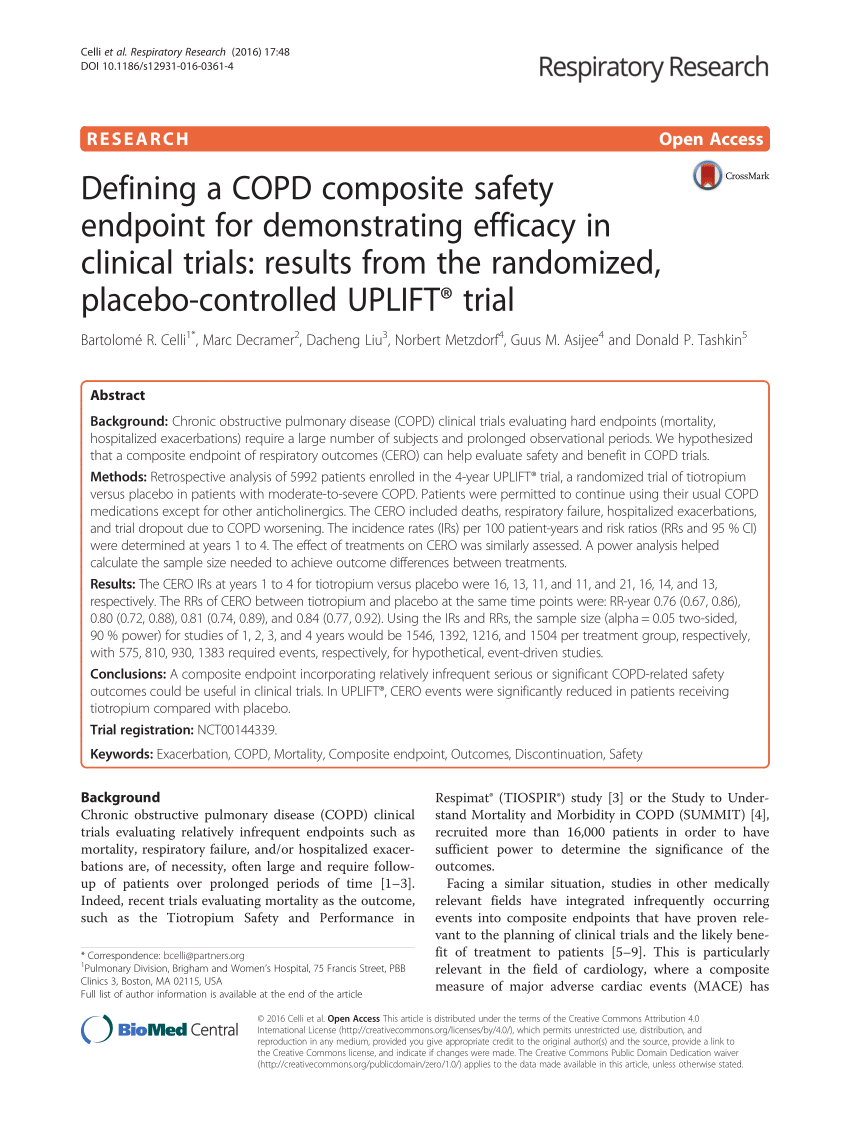
PDF) Defining a COPD composite safety endpoint for demonstrating efficacy in clinical trials: Results from the randomized, placebo-controlled UPLIFT® trial

Challenging Issues in Clinical Trial Design: Part 4 of a 4-Part Series on Statistics for Clinical Trials - ScienceDirect


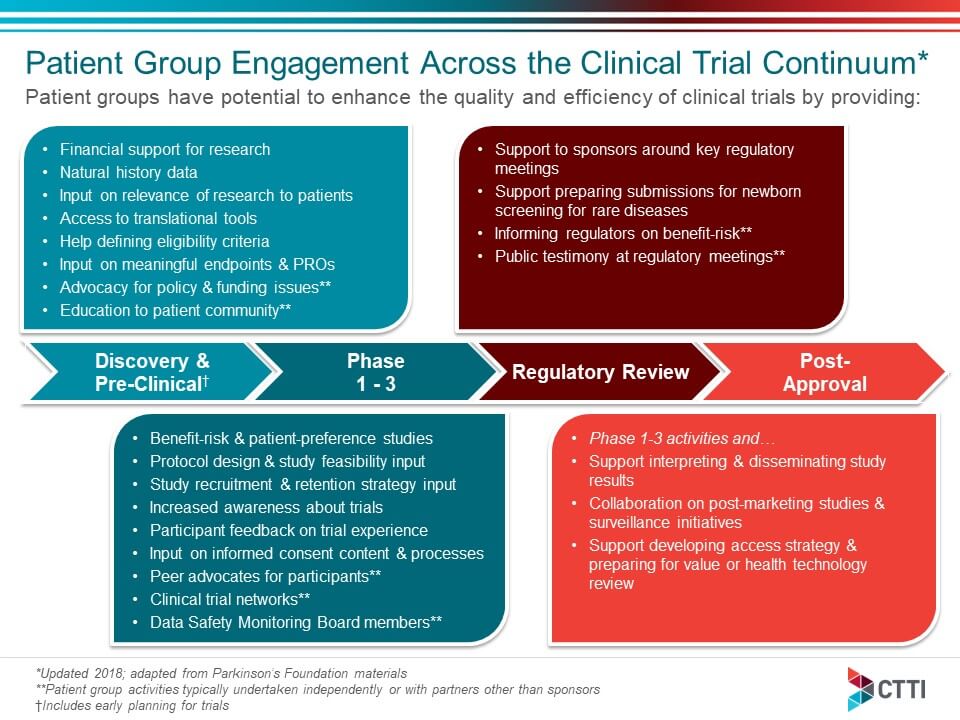
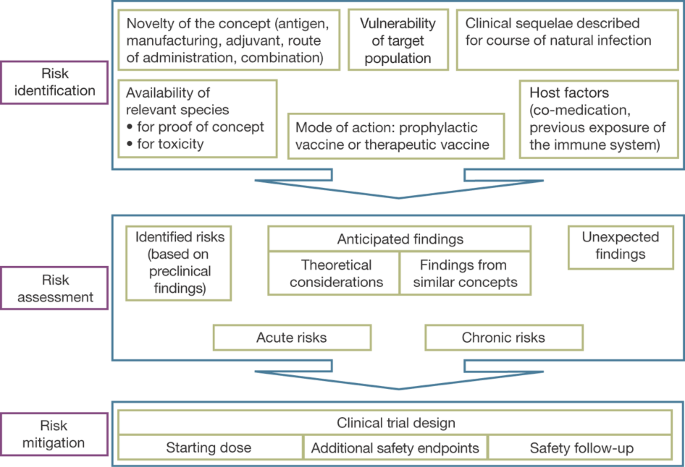


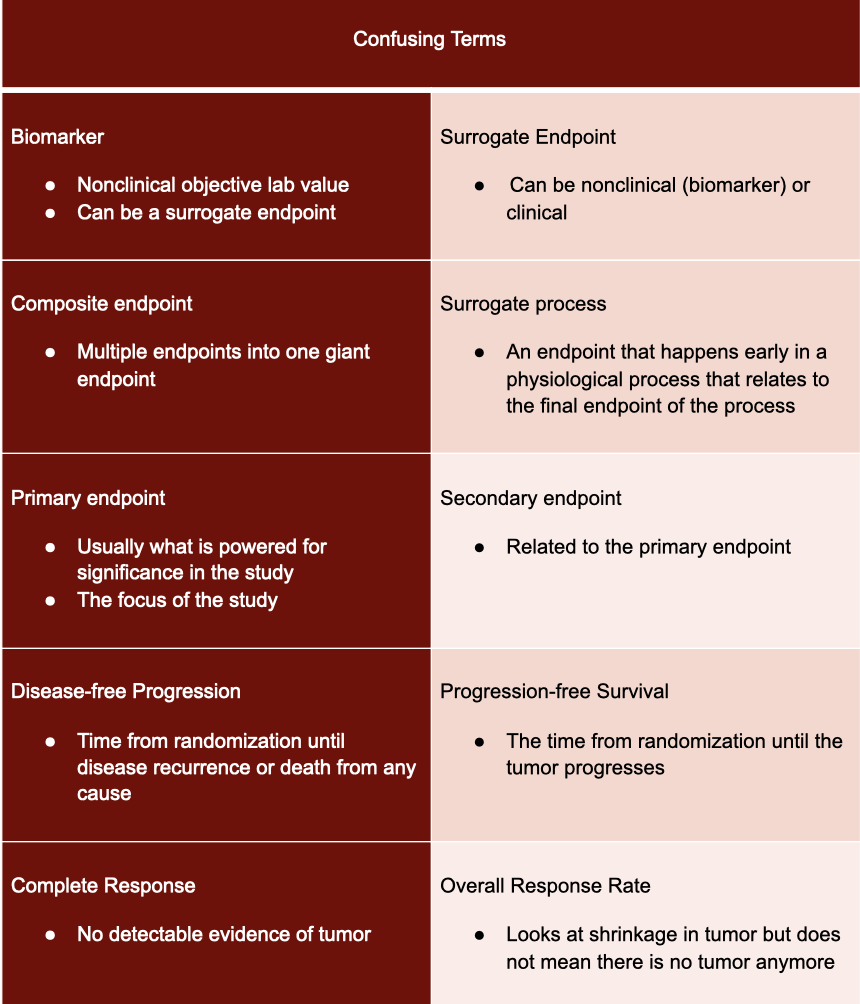

![Stages of PWS Drug Development Overview [VIDEO] Stages of PWS Drug Development Overview [VIDEO]](https://www.fpwr.org/hubfs/Clinical%20Trials/Drug%20Development%20Process/Slide5.jpeg)