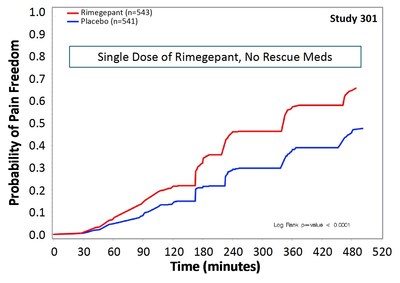
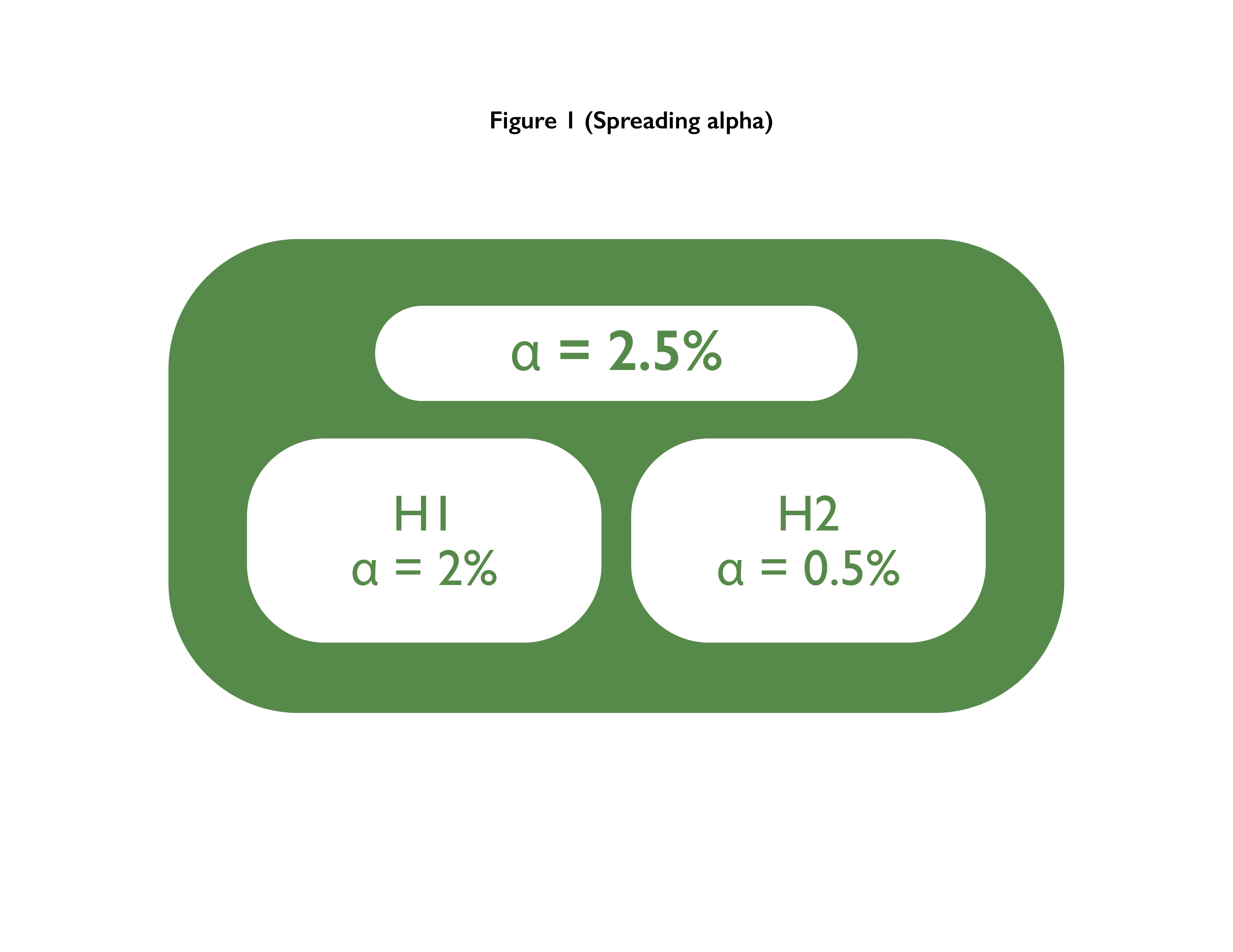
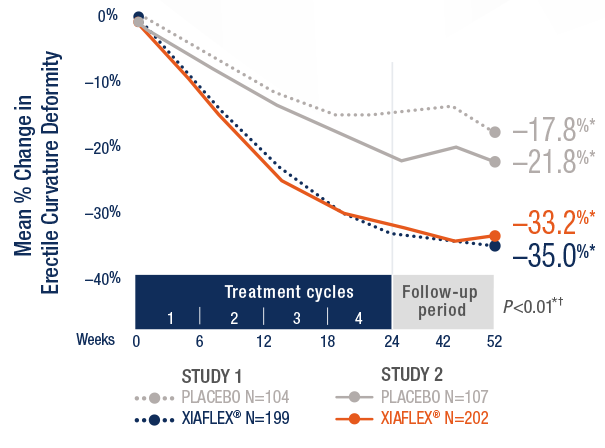
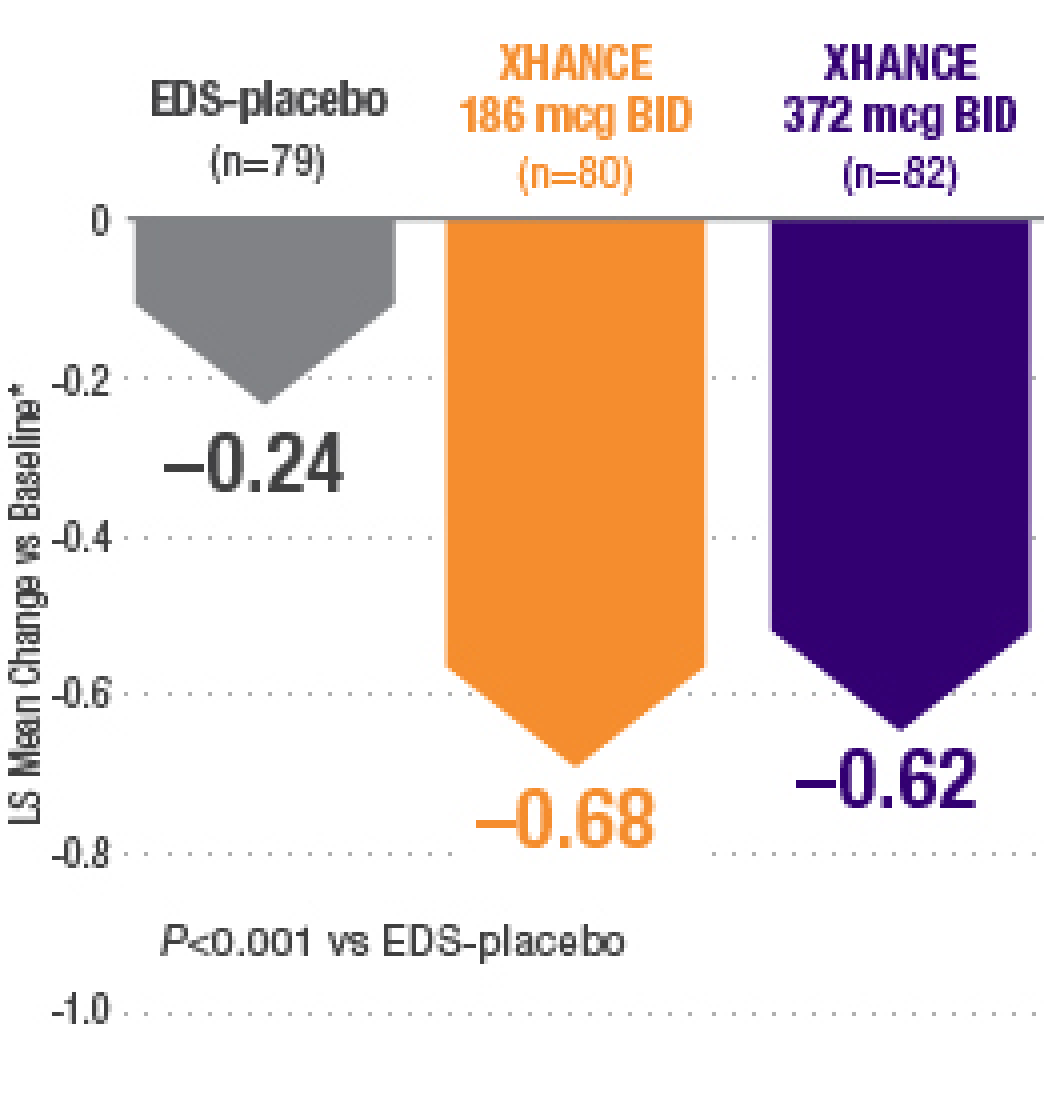
Defining Endpoints and Biomarkers in Inflammatory Bowel Disease: Moving the Needle Through Clinical Trial Design - Gastroenterology
A study design with conditional, serially assessed coв•'primary endpoints: An application to a singleв•'arm, pilot non
Rationale and design of a prospective substudy of clinical endpoint adjudication processes within an investigator-reported rando

Pfizer Shares Co-Primary Endpoint Results from Post-Marketing Required Safety Study of XELJANZ® (tofacitinib) in Subjects with Rheumatoid Arthritis (RA) | Business Wire

Impact of non‐binding FDA guidances on primary endpoint selection in Alzheimer's disease trials - Yu - 2022 - Alzheimer's & Dementia: Translational Research & Clinical Interventions - Wiley Online Library

Choice of Primary (or Co-primary) Endpoints: Efficacy, Safety, or Net Clinical Benefit in Superiority and Non-inferiority Trials | tctmd.com
Sample size determination for a specific region in multiregional clinical trials with multiple co-primary endpoints

Primary and Secondary Outcome Reporting in Randomized Trials: JACC State-of-the-Art Review | Journal of the American College of Cardiology

Metastases-directed Radiotherapy in Addition to Standard Systemic Therapy in Patients with Oligometastatic Breast Cancer: Study protocol for a randomized controlled multi-national and multi-center clinical trial (OLIGOMA) - Clinical and Translational ...
Sequential sample size re-estimation in clinical trials with multiple co-primary endpoints - WRAP: Warwick Research Archive Portal
Multiple Co-primary Endpoints: Medical and Statistical Solutions A Report From the Multiple Endpoints Expert Team of the Pharmac