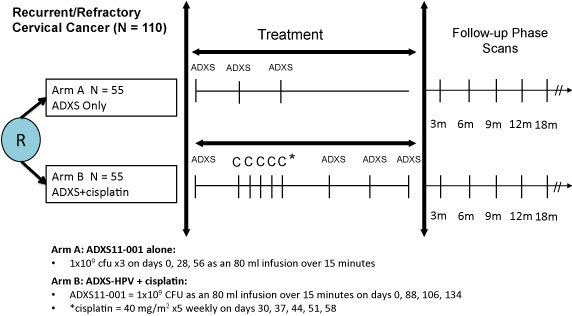
PDF) A Randomized Phase 2 Study of ADXS11-001 Listeria monocytogenes–Listeriolysin O Immunotherapy With or Without Cisplatin in Treatment of Advanced Cervical Cancer
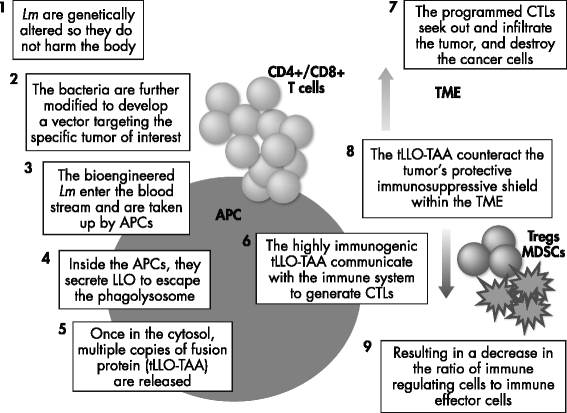
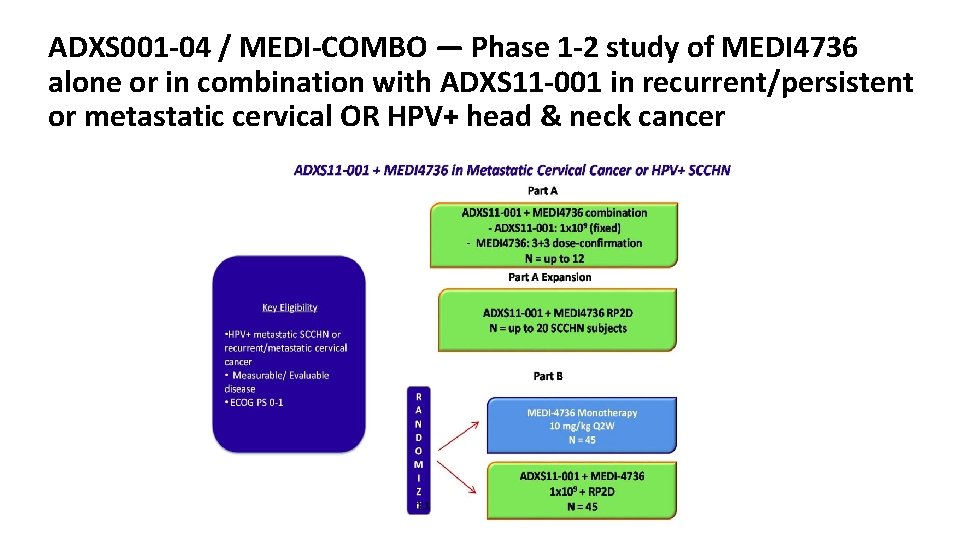
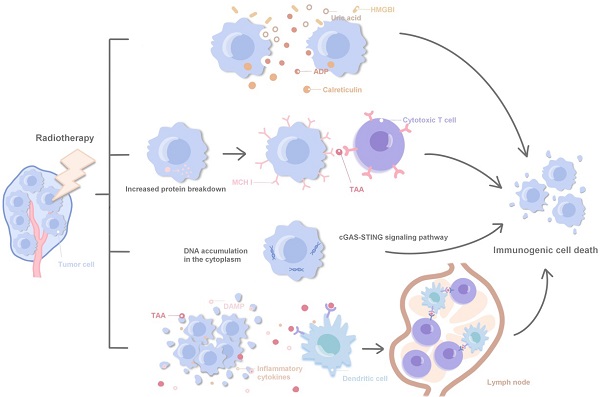
Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy | Gynecologic Oncology Research and Practice | Full Text
ADXS11-001 immunotherapy in squamous or non-squamous persistent/recurrent metastatic cervical cancer: Results from stage 1 [and

The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). - Abstract - Europe PMC
Tolerability of ADXS11-001 Lm-LLO Listeria-Based Immunotherapy With Mitomycin, Fluorouracil, and Radiation for Anal Cancer
PDF) High-dose treatment with ADXS11-001, a listeria monocytogenes (Lm)-listeriolysin O (LLO) immunotherapy, in women with cervical cancer
ADXS11-001 immunotherapy in squamous or non-squamous persistent/recurrent metastatic cervical cancer: Results from stage 1 [and
P2 study of ADXS11-001 Immunotherapy in patients with persistent/recurrent, surgically unresectable locoregional, or metastatic
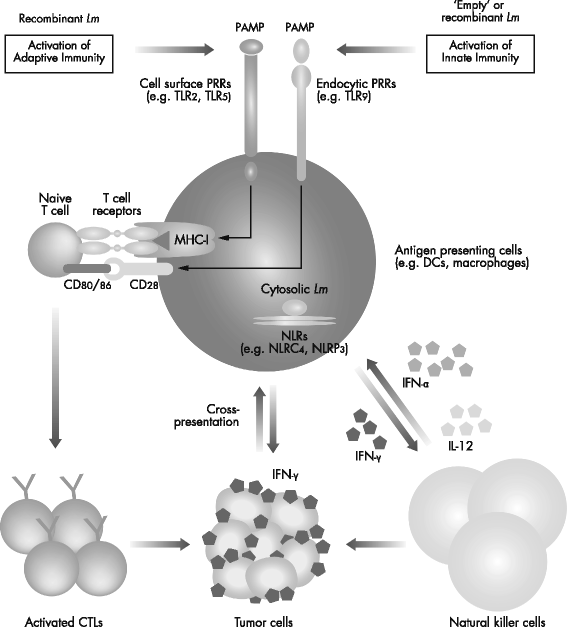
Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy | Gynecologic Oncology Research and Practice | Full Text

PDF) A Randomized Phase 2 Study of ADXS11-001 Listeria monocytogenes–Listeriolysin O Immunotherapy With or Without Cisplatin in Treatment of Advanced Cervical Cancer

Phase II study of axalimogene filolisbac (ADXS-HPV) for platinum-refractory cervical carcinoma: An NRG oncology/gynecologic oncology group study - ScienceDirect

PDF) Systemic listeriosis following vaccination with the attenuated Listeria monocytogenes therapeutic vaccine, ADXS11-001
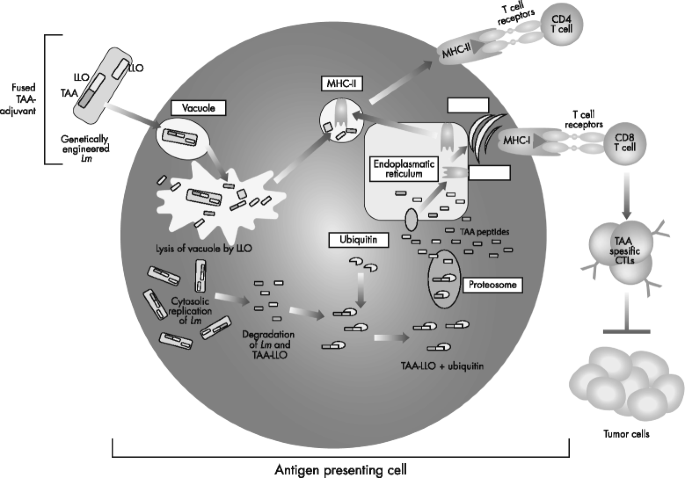
Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy | Gynecologic Oncology Research and Practice | Full Text

A phase II trial of the Wee1 inhibitor adavosertib (AZD1775) in recurrent uterine serous carcinoma - Gynecologic Oncology

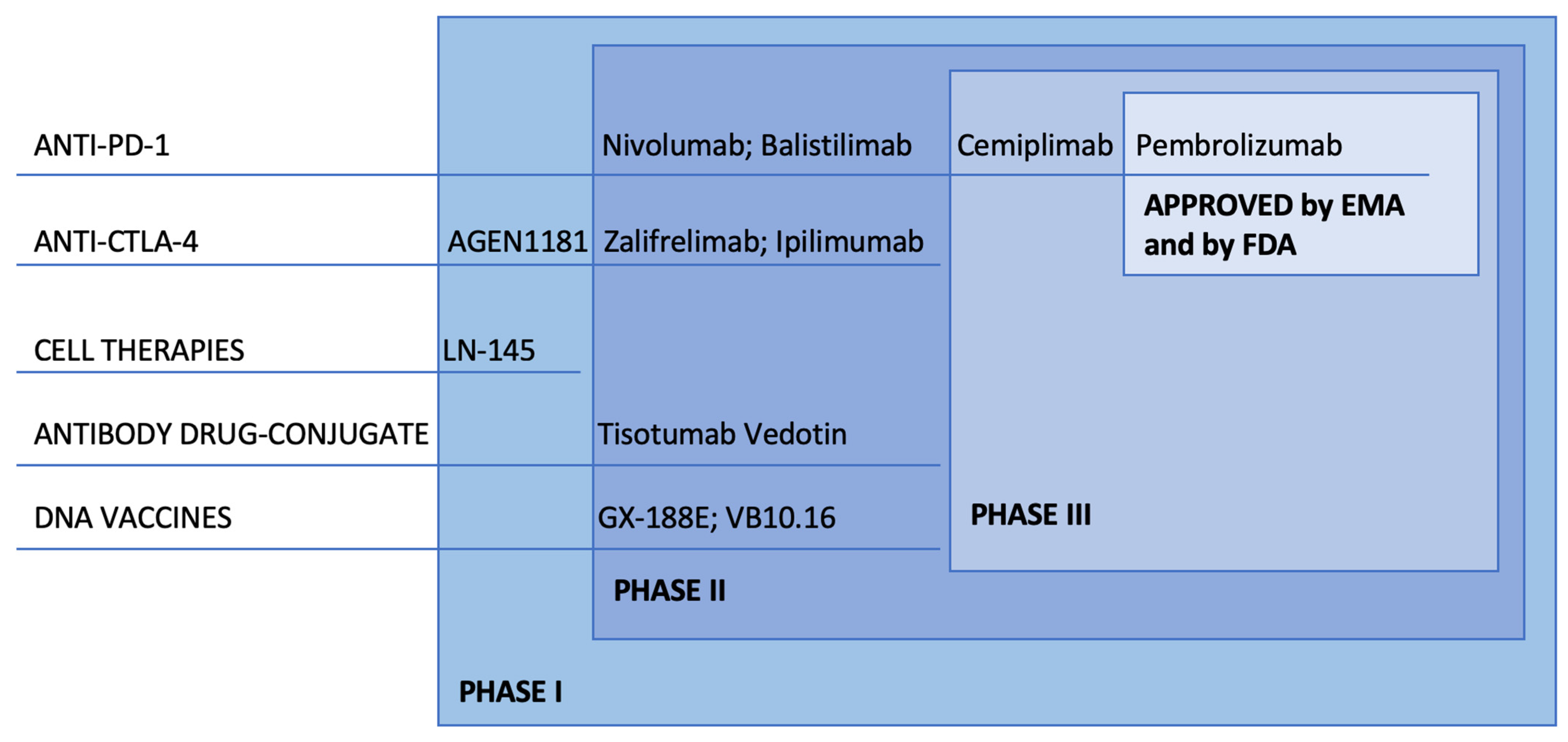

%20for%20Carcinoma,%20Squamous%20Cell.png?md=1)


![PDF] Immunotherapy for Uterine Cervical Cancer | Semantic Scholar PDF] Immunotherapy for Uterine Cervical Cancer | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/397156ac0767e149384fd3767cfbe8adcbd994c0/7-Table3-1.png)